-
-
Catalog Numbers for Ordering Reagents:
-
Proteintech FlexAble 2.0 Kit and Antibodies
-
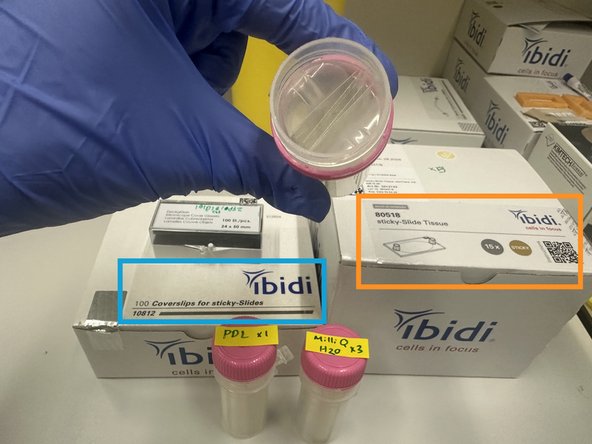
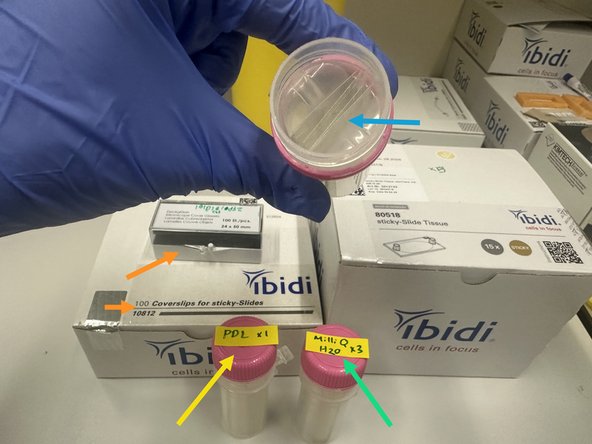
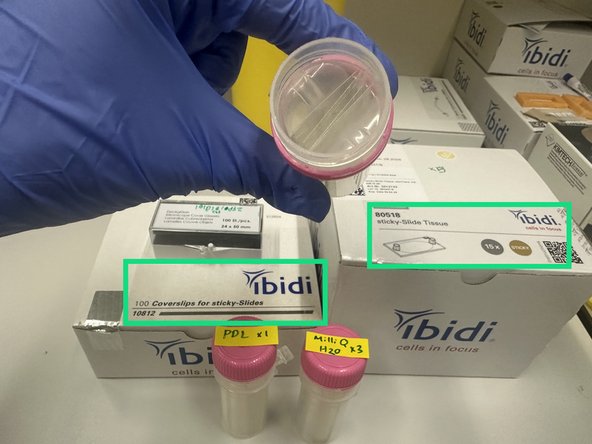
Ibidi Coverslips (cat# 10812)
-
Coating Reagents- Chrome Alum Gelatin (Newcomer Supply, cat no. 1033A USA) *important for tissue adherence for multiplex cycles, for adherent cells can omit. Substitution Poly-D-Lysine (cat#P6407 Sigma) or Poly-L-Lysine (cat# P8920 Sigma 0.1% w/v) coating also possible
-
Ibidi Sticky Slides for Tissue (cat# 80518)
-
15mL Falcon Tubes (cat# 10773501) Thermofisher. Important to match thread size of the Aria pump.
-
Connectors: https://www.microfluidic-chipshop.com/ Tube Tuck Adapter Luer 1/16" Fluidic Design: 1581
-
-
-
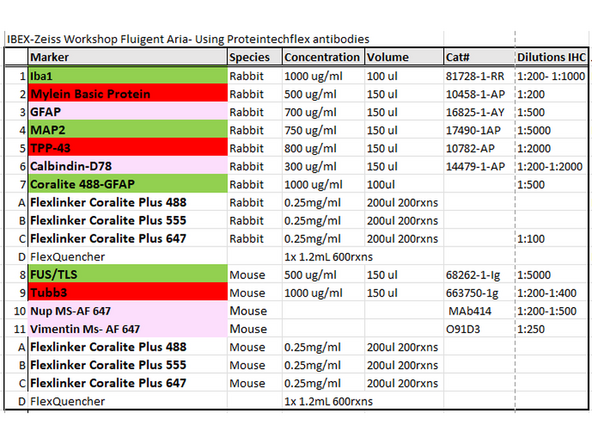
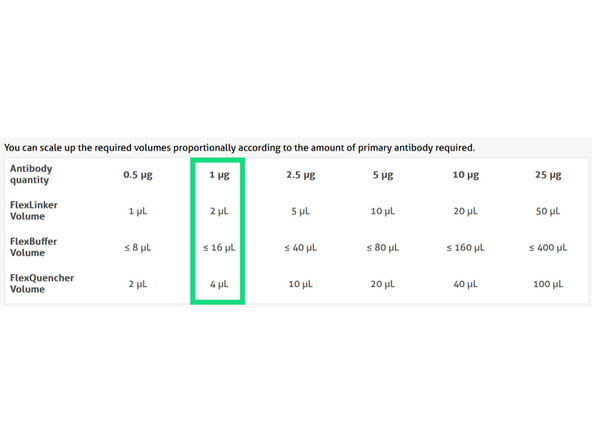
Proteintech- Antibody Labeling with FlexAble 2.0 Kit. Use 1ug of antibody: Proteintech FlexAble calculator
-
Assign Antibody to Flexlinker Coralite Plus Flurophore (i.e. 488, 555, or 647).
-
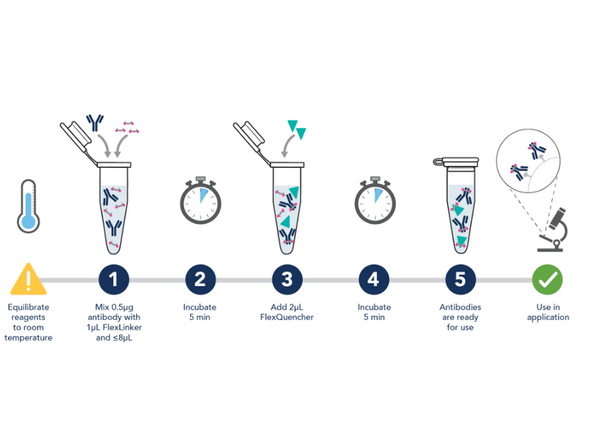
1. Combine 1.0 ug of primary antibody (i.e, 1.4uL of GFAP 700ug/mL Cat#16825-1AY) with 2ul of FlexLinker Coralite Plus 555. Add 12.6uL of flexBuffer to bring total volume to 16uL.
-
2. Mix gently and icubate for 5 minutes at room temperature in the dark (in opaque microcentrifuge tubes).
-
3. Add 4uL of FlexQuencher.
-
4. Mix gently and incubate for 5 minutes at room temperature in the dark.
-
The Antibody is now ready to use- Store at 4 degrees.
-
-
-
Take Protocol of Choice: One here or steps below.
-
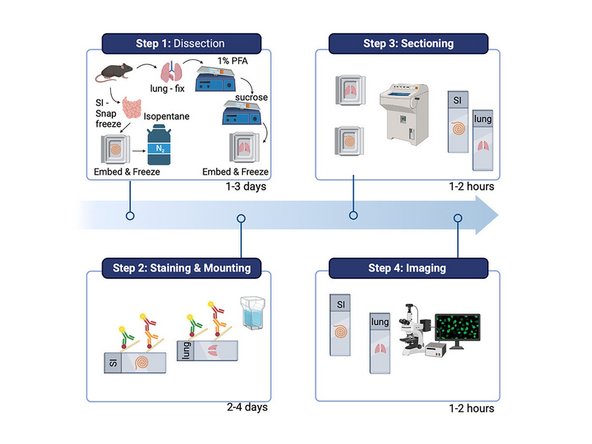
1. Perfusion with PBS followed by 4%PFA, until tissue appears white.
-
2. Post Fix for 1hr in 4% PFA (adjust time to tissue size).
-
3. Rinse 3x with PBS, and soak in Sucrose 30% overnight.
-
4. Fill mold with OCT, and position tissue in mold mindful of orientation for future cryosectioning.
-
5. Freeze in in finely crushed dry ice, wait for OCT to become opaque, before moving and storing at -80 before cyrosectioning.
-
Always acclimate block to cryostat temperature before sectioning.
-
-
-
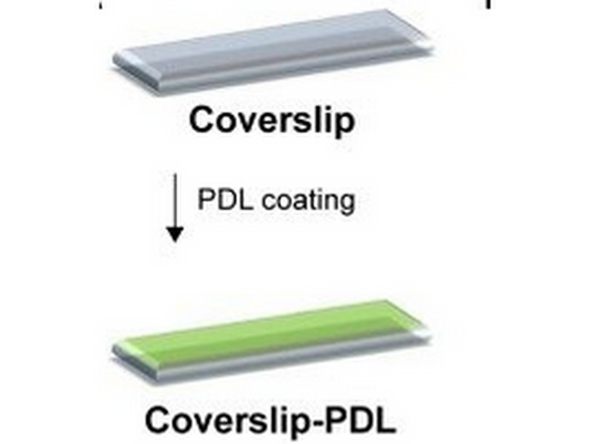
IBEX- Recommended to coat in Chrome Alum Gelatin
-
Substitution Poly-D-Lysine (cat#P6407 Sigma) or Poly-L-Lysine (cat# P8920 Sigma 0.1% w/v) coating also possible
-
Poly D Lysine (PDL) Sigma Aldrich- P6407-5mg- substitute for original publication Chrome Alum Gelatin (Newcomer Supply, cat no. 1033A USA) not available in EU or CH. *important for tissue adherence for multiplex cycles, for adherent cells can omit.
-
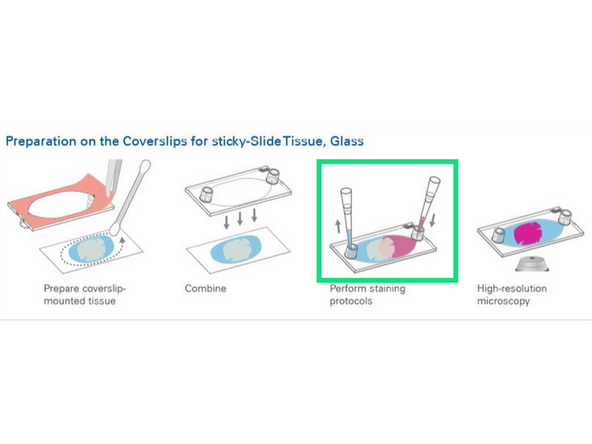
1. Ibidi Glass Coverslips for sticky slides.Cat# 10812 #1.5H Glass Coverslip 25mm x 75 mm
-
2. Coat surface (marking coated side) or submerge in coating. Remove excess liquid (can be used for coating subsequent coverslips)
-
3. Rinse 3x with dH2O
-
4. Dry for at least 2 hours
-
5. Ready to section onto. Do not freeze once coated.
-
-
-
1. Section at 10 microns. Or ideal section thickness for tissue and microscope of choice acquisition.
-
2. Collect sections onto PDL coated (or alternative coating) Ibidi coverslips.
-
3. Collect extra region of interest sections on several superfrost plus slides, for validating antibody panel prior to multiplexing experiment.
-
4. Store at 4 degrees do not freeze until ready for mounting on Ibidi Sticky slides.
-
-
-
1. Prepare Blocking Buffer from 1X PBS (Gibco, cat no. 10010-023) containing 0.3% Triton-X-100 (Millipore Sigma, cat no. 93443), 1% Bovine Serum Albumin (Millipore Sigma, cat no. A1933) and
-
*1:100 dilution of Human (BD Biosciences, cat no. 564219) or Mouse (BD Biosciences, cat no. 553141) Fe-block or TrueStain FcX (BioLegend, cat no. 101320). • *ZMB omit the Fe in the block.
-
2. Prepare Antibody Dilutant from 1X PBS, with 0.1% BSA.
-
3. Prepare Antibodies with suggested dilutions.
-
4. Block test sections on Superfrost Plus slides, in humidified chamber for 20min-1hr.
-
5. Leave Antibody on slide for 2hrs at RT, or overnight at 4 degrees.
-
6. Test Exposure settings and verify that antibody dilutions are correct for your tissue of interest.
-
Once confirmed correct staining of single cycles from your panel on slide use same dilutions for your Fluigent Aria Multiplexing Protocol in combination with other cycles.
-
-
-
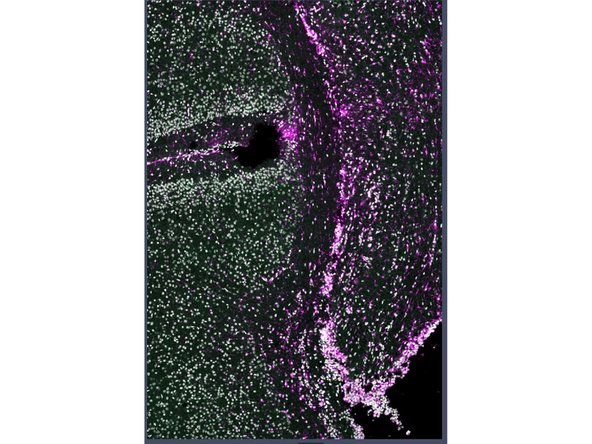
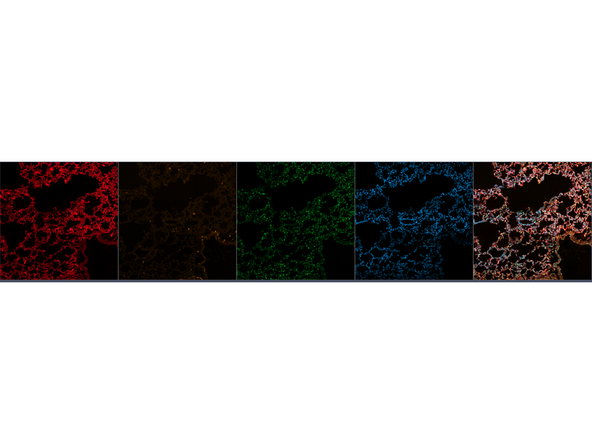
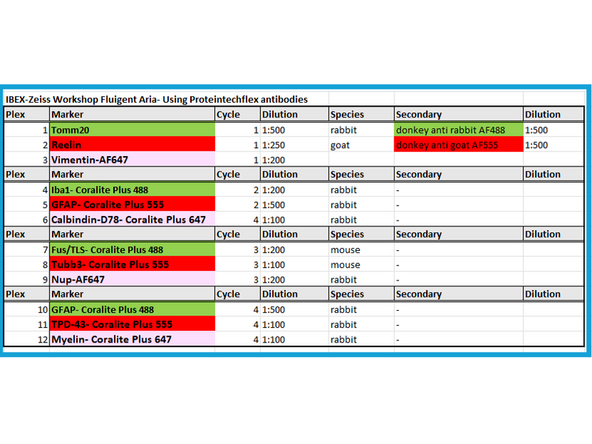
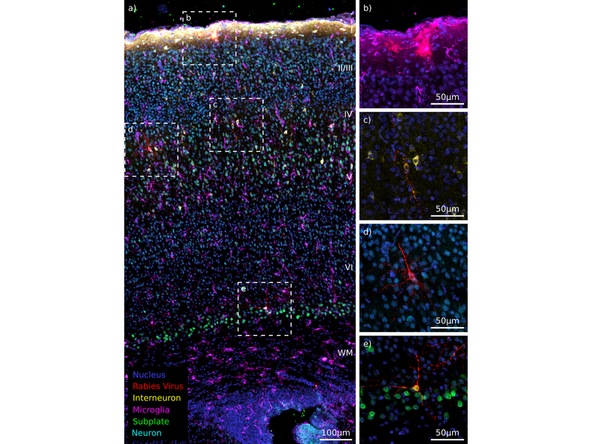
Example Panel for IBEX Multiplexing Experiment on Neuronal Tissue
-
Indirect Labeling- Primary and Secondary Antibodies can only be used in Cycle 1 in IBEX
-
Direct Labeled Antibodies- Conjugated Primaries can be used in all other Cycles (Cycle 2- Cycle# n) or omit indirect labeled in Cycle 1 and use conjugated for all IBEX cycles.
-
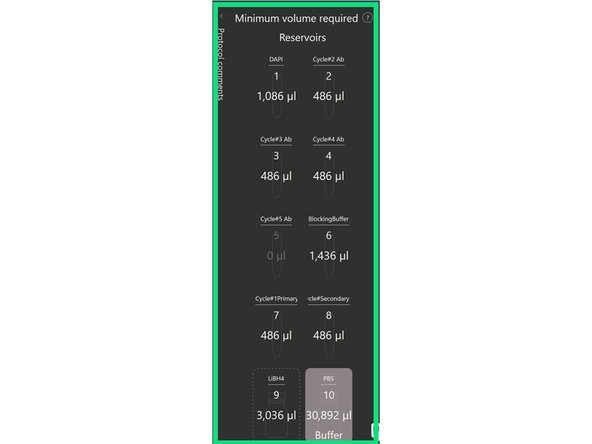
Load Reagents (1-8) in 15mL Falcons at correct dilutions and add freshly filtered LiBH4 and PBS to the 100mL glass bottles in reservoirs #9 and #10 respectively, label in Aria software.
-
Prepare the following buffers: 1X PBS, Blocking Buffer (1% BSA in 1X PBS), Antibody Dilutient (0.1%BSA in 1XPBS), Lithium Borohydrate (LiBH4_
-
Critical: When you prepared LiBH4 in diH20 do so in a hood and make a 1 mg/ml solution at least 5ml at a time but never more than 10mL. Leave to~incubate at room temperature for 10min, after gentle mixing. Pass the solution through a 0.22 μm syringe filter to remove any impurities.
-
Caution: Perform the LiBH4 preparation steps in a chemical hood with appropriate PPE. The reaction can generate hydrogen gas, which is flammable. To avoid flames, always work with small amounts (<10 mg) of Li8H4.
-
Store LiBH4 in a sealed container with desiccant and use a new vial of LiBH4 no later than 4 weeks after opening original container.
-
-
-
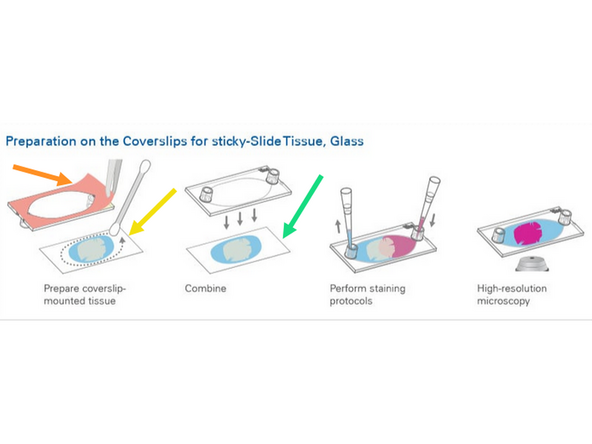
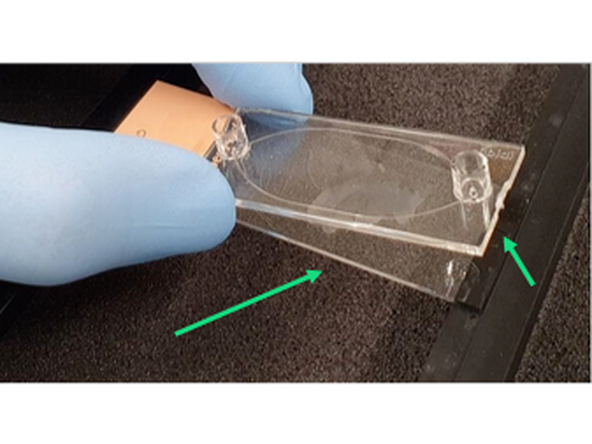
1. Pull away Ibidi Sticky slide film to reveal sticky coating
-
2. Clean excess tissue or folded sections away and make dry surface for applying sticky slide to glass coverslip
-
3. Align Ibidi 25mm x 75mm coverslip (cat# 10812) with Ibidi sticky slide (cat# 80518) carefully.
-
Do not block ports with tissue and carefully align so no over or under hangs of the glass coverslip.
-
-
-
Fill Chamber with PBS or TBS and avoid trapping air bubbles.
-
Give time for OCT from sections to dissolve and tissue to acclimate to buffer.
-
Tap away any bubbles in chamber before proceeding with Multiplex Experiment using Fluigent Aria Pump
-
-
-
1. Attach blue connector to inlet (ChipShop catalog #1581 Tube Tuck Adapter Luer 1/16")
-
2. Attach elbow clear connector to waste output outlet
-
Can manually use Fluigent Aria pump (not with software, but with Aria control panel on pump) to load fresh buffer and confirm no bubbles or leaks.
-
3. Feed tubing through microscope door
-
4. Mount sticky slide-tissue coverslip into slide carrier to load in the microscope, within the microscope.
-
5. Check that no tubing interferes with stage movement or acquisition.
-
6. Set up slide overview and acquisition settings as in this guide: Fluigent Aria 3: Combine microfluidics with ZEISS microscopes
-
-
-
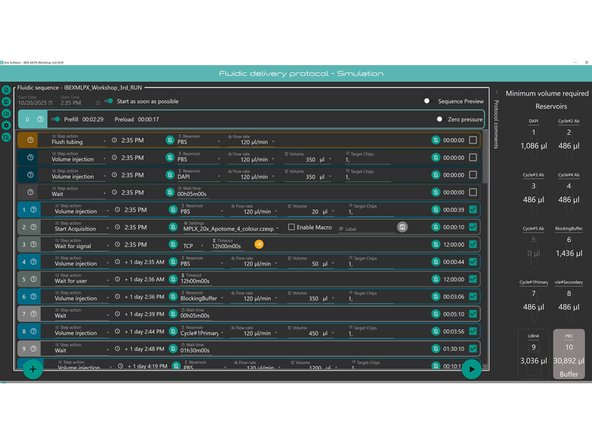
1. Setup Aria Protocol for Multiplexing Protocol of Choice- with your Reagents.
-
Guide here for writing an Aria Protocol- Fluigent Aria 2: How to write an Aria Sequence
-
Guide here for starting the Aria Pump- Fluigent Aria 1: Startup
-
Guide here for Aria to Zeiss Microscope- Fluigent Aria 3: Combine microfluidics with Zeiss Microscopes
-
Set region of interest of your tissue section
-
-
-
Evaluate results from your run.
-
Optimize. 1. Dilutions of Antibodies 2. Placement of tissue 3. Positioning of Antibodies in Cycles. 4. Bleaching strategy (IBEX) or Elution strategy (4i) of antibodies from each cycle
-
-
Run a real Multiplexing experiment!
-
Almost done!
Finish Line