Introduction
How to start up and mount your first sample on the Leica SP8 confocal laser scanning microscope located at the IPZ at Institute of Parasitology, Room PV-10.55.
Please find detailed information about the system setup here.
-
-
The microscope stand is always on for stability reasons. Never turn it off.
-
The corresponding microscope control box can be found underneath the microscope table.
-
Switch ON the fluorescence lamp.
-
Switch ON the "Scanner Power", "Laser Power", and turn the "Laser Emission" key to "On-1" (control unit underneath the table).
-
Switch ON the power knob (on the PC table).
-
-
-
Sign-in with your ZMB core credentials.
-
-
-
Start the "LAS X" software.
-
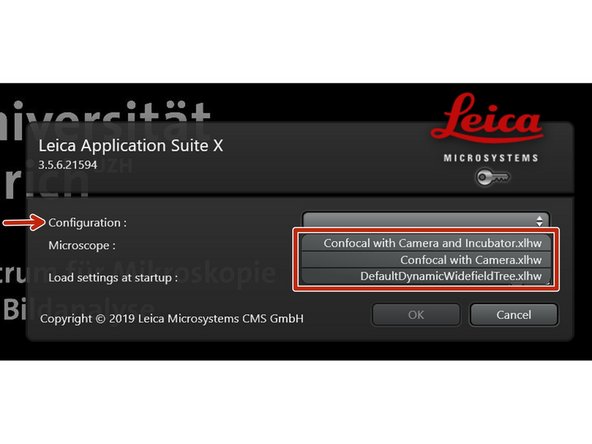
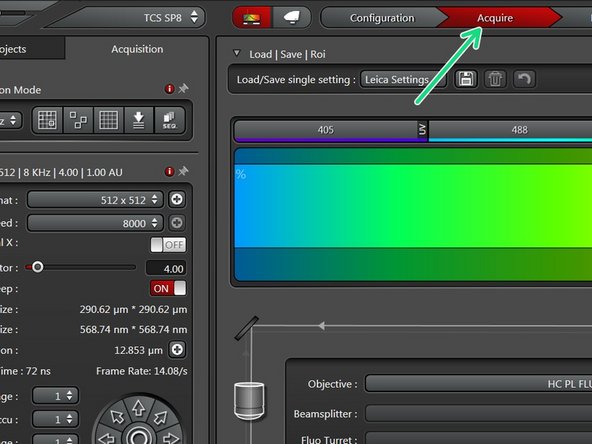
Select the appropriate "Configuration".
-
"Confocal with Camera and Incubator.xlhw" if environmental control is needed. Make sure the needed components have been switched on.
-
"Confocal with Camera.xlhw" for standard room temperatur (RT) measurements.
-
"DefaultDynamicWidefieldTree.xlhw" for only widefield/camera option.
-
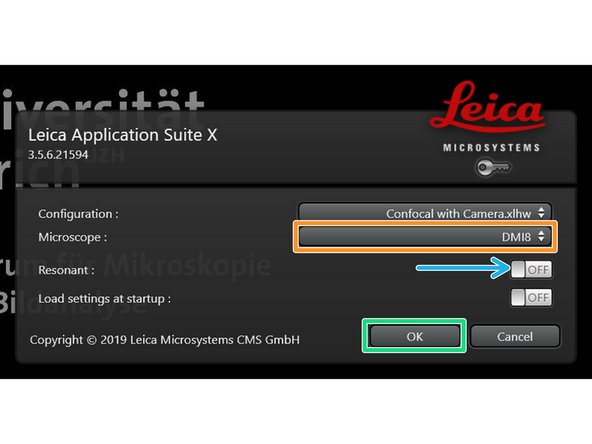
Make sure "DMI8" is selected as "Microscope".
-
Select either "Resonant" (ON) or non-"Resonant" (OFF) scanning mode.
-
Click "OK".
-
-
-
Please note: the x/y stage at this microscope does not need any initialization to function!
-
The indicator light "MOTOR ON/OFF" at the stage (left hand side) must be green continuously.
-
Flashing green light at the stage indicates the stage was disengaged by touching or manual movement. Press the green light button to activate it again.
-
-
-
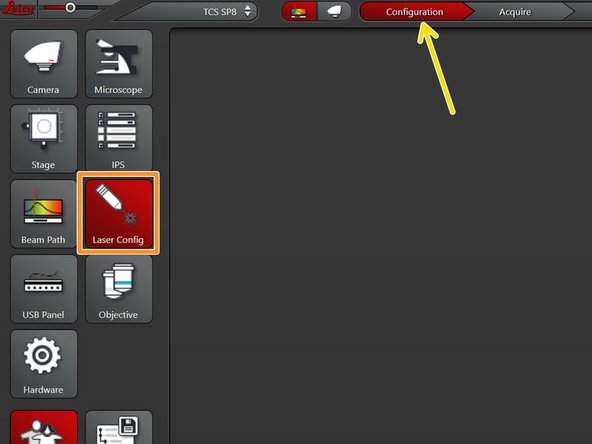
Go to "Configuration".
-
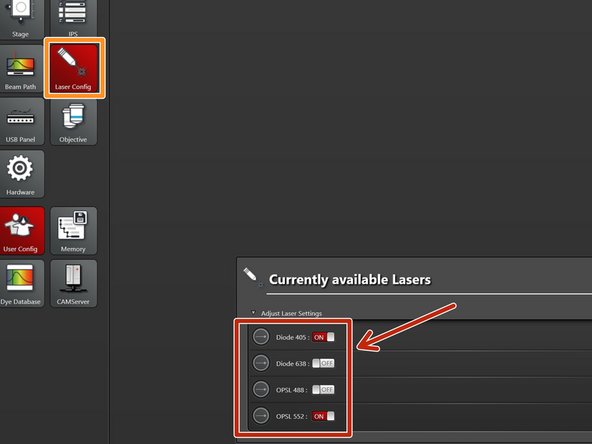
Select "Laser Config".
-
Switch "ON" the lasers you will need.
-
Go back to "Acquire".
-
-
-
Lower the objective turret by pressing the downwards "Z" button on the right side of the microscope.
-
This is a mandatory step as it avoids possible collision of the objectives and stage during exchange of inserts and/or samples.
-
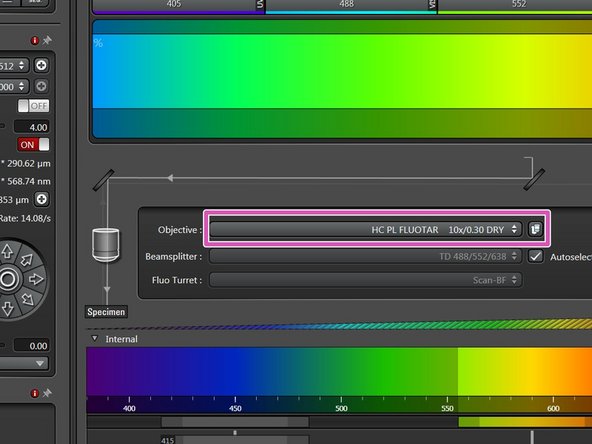
You can now toggle between objectives within the software (drop-down menu).
-
Select the 10x dry objective.
-
In order to facilitate the focusing it is recommended to start with the 10x dry objective.
-
-
-
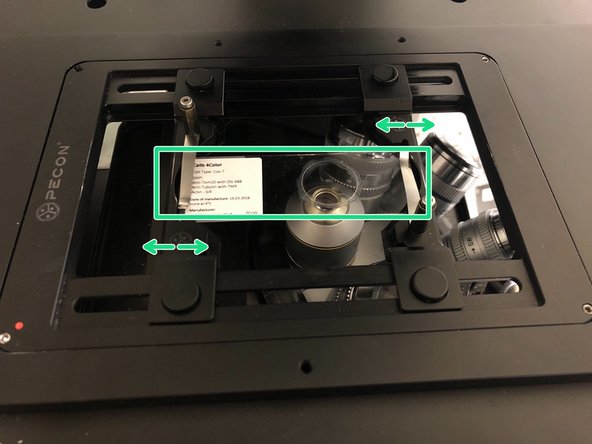
You will find different stage inserts in the box on the shelf.
-
Push the condensor arm of the microscope to the back and install the chosen sample holder.
-
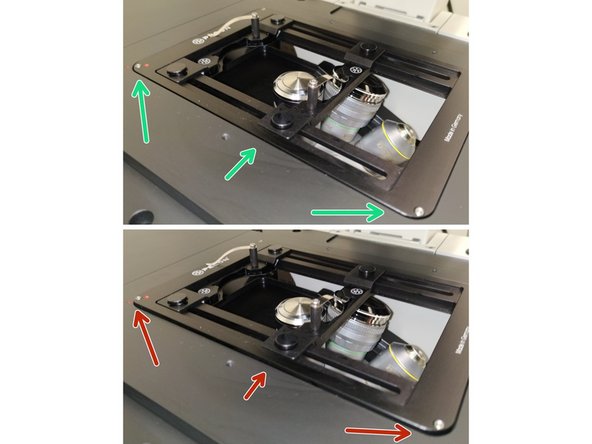
Make sure that the stage insert is correctly inserted and flat.
-
Here correct and flat.
-
Here not inserted correctly (stage not flat and shaky).
-
The stage might disengage while installing the stage insert. Press the flashing green light button again as already mentioned previously.
-
-
-
Insert your sample with the coverslip facing down.
-
Adjust the variable clamping range and moveable brackets to properly fix your sample.
-
Move your sample over the objective with the help of the Joystick.
-
With the little slider knobs on both sides of the lower wheel you can change between fast and slow movement.
-
Fast movement - pressing the knobs downwards.
-
Slow movement - pulling the knobs towards you.
-
Bring back condenser arm to its straight position.
-
-
-
On the touch screen at the microscope choose the light path tab.
-
Click "FLUO" and choose an appropriate "FLUO-Filtercube" e.g. "DAPI".
-
Open the "IL-Shutter" (if actived the dot is yellow).
-
Look through the oculars and focus your sample by using:
-
the focus wheel on the microscope stand,
-
or the z-wheel on the external controller (Smart Move).
-
Moving objectives upwards (towards sample) turn z-wheels clockwise/away from you. Moving objectives downwards (away from sample) turn z-wheels counter-clockwise/towards you.
-
Toggle between "Z FINE" and "Z COARSE" directly on the Smart Move.
-
-
-
The storage of the focal plane is helpful in order to find the focus back if the sample or objective will be changed.
-
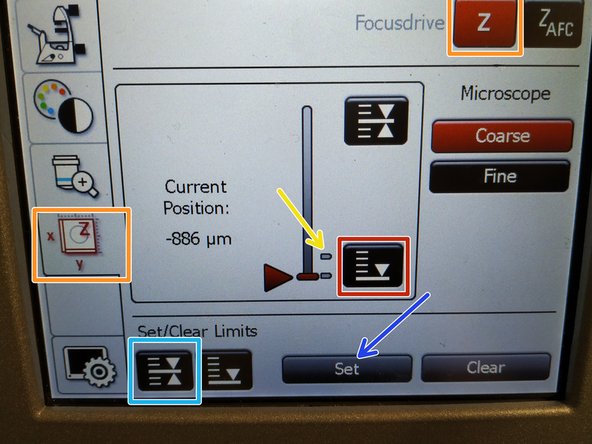
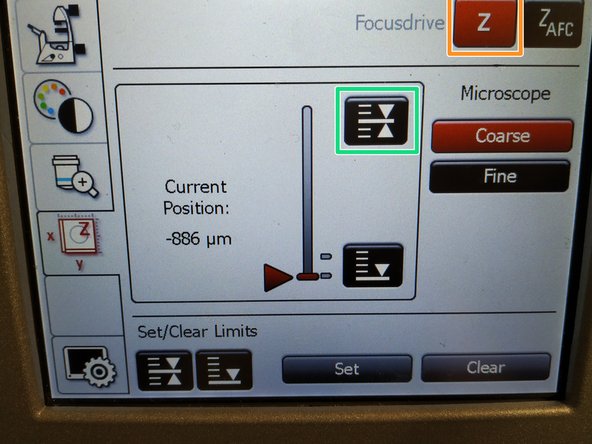
To save your current focus position select the "xyz tab" and the "Focusdrive Z" on the touchscreen of the microscope.
-
Click the "Upper Focus Limit" button.
-
Press "Set".
-
If done successful you will see an upper marker line appearing.
-
Press the "Lower Limit" button in order to move down (for safe change of the objective or the sample).
-
-
-
Remove your sample and toggle within the software to the objective of choice.
-
Depending on the objective different immersion media will be used. Apply either on the sample or directly to the objective.
-
Oil objectives: "Type-F" immersion liquid.
-
"Glycerin" objectives: either "Type-G" immersion liquid (for RT measurements) or "Glycerin" immersion liquid (for measurements at 37°C).
-
"Water" objectives: ddH2O (always use fresh).
-
Please further consider the additional information in the next step to guaranty proper image acquisition.
-
Mount your sample again and press the "Upper Focus Limit" button.
-
Focus your sample as described previously.
-
-
-
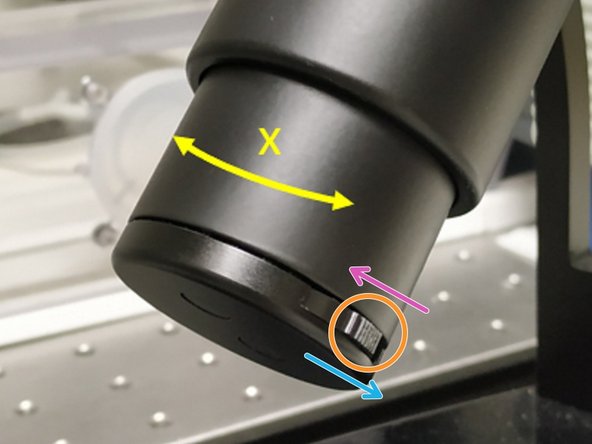
For some objectives the correction collar has to be adjusted.
-
20x IMM (multi-immersion - Oil, Glycerin or Water) needs to be set to the corresponding immersion media ("OIL", "GLYC" or "0.17-W" (with cover glass) or "W-0" (without cover glass)).
-
63x water you can correct for the cover glass thickness (0.14-0.18 mm). Standard is usually 0.17 mm.
-
Make sure that the cap of the spring-loaded front lens is released (working position).
-
Please, DO NOT remove the objectives for adjustment. They can be also accessed on the system.
-