Introduction
In this guide of the Center for Microscopy and Image Analysis we show how to start up and mount your sample on the Leica SP8 inverse confocal laser scanning microscope located at the Institute of Medical Microbiology.
Please find detailed information about the system setup here.
-
-
Switch ON the fluorescence lamp.
-
Once turned on, the lamp should remain ON for a minimum of 30 min.
-
On the right hand side of the table (main switch board):
-
Switch ON the "PC /Microscope", "Scanner Power" and "Laser Power".
-
Turn the "Laser Emission" key to "On-1".
-
-
-
For certain configurations in the "LAS X" software the Camera is needed to be switched on for the start-up.
-
Switch ON camera.
-
First an orange LED will light up, and will turn green when fully warmed up.
-
-
-
Insert wisdom here.
-
-
-
Sign-in with your ZMB core credentials.
-
-
-
Start the "LAS X" software.
-
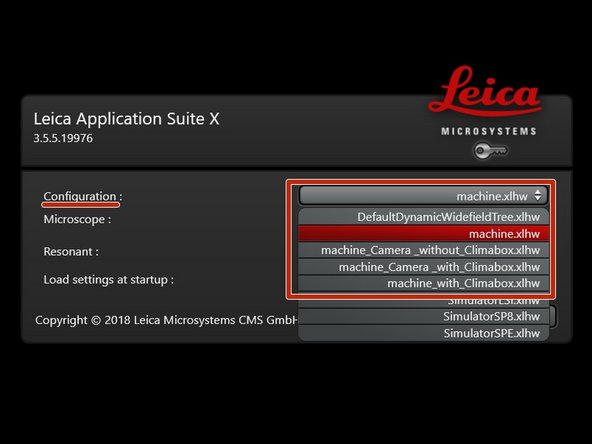
Select the appropriate "Configuration":
-
"machine.xlhw" - standard confocal measurements.
-
"machine_Camera_without_Climabox.xlhw" - confocal as well as widefield option. Make sure Camera is switched ON.
-
"machine_Camera_with_Climabox.xlhw" if environmental control is needed. Make sure the needed components (Camera, Cube, Brick) have been switched ON.
-
"machine_with_Climabox.xlhw" if environmental control is needed without widefield option. Make sure the needed components (Cube, Brick) have been switched ON.
-
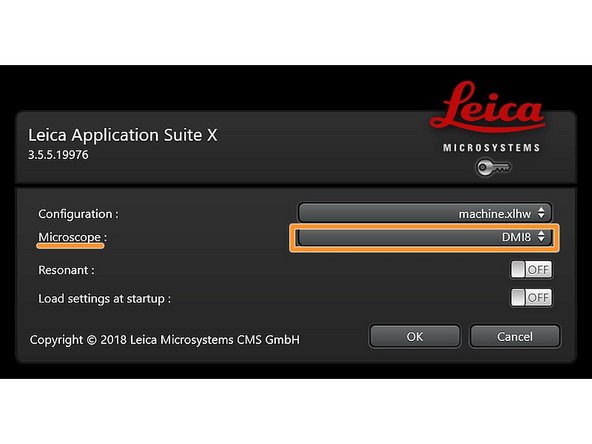
Make sure "DMI8" is selected as "Microscope".
-
-
-
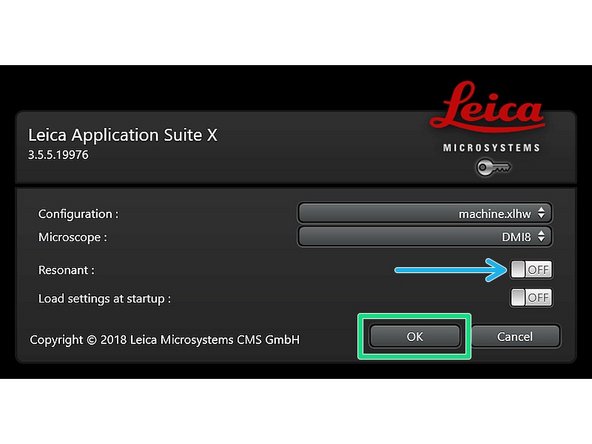
Select either "Resonant" (ON) or non-"Resonant" (OFF) scanning mode.
-
Click "OK".
-
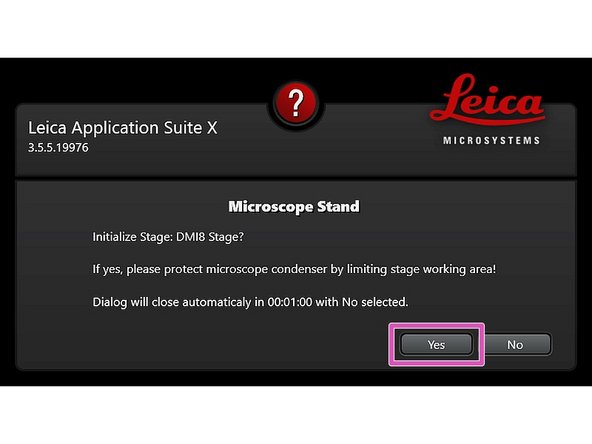
Ensure that no sample is mounted on the stage and click "YES" to initialize the x/y stage.
-
x/y stage initialization is necessary to be able to use the LAS X Navigator function.
-
-
-
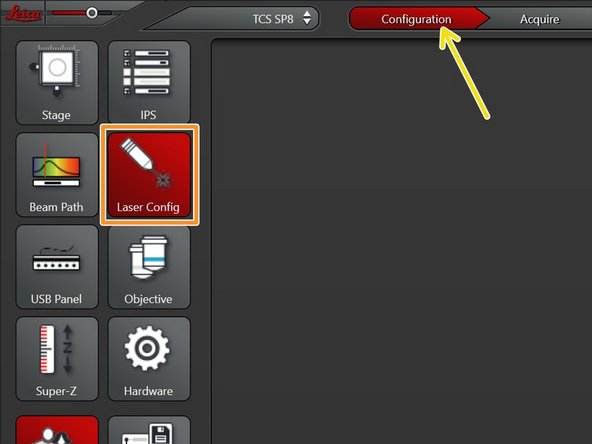
Go to "Configuration".
-
Select "Laser Config".
-
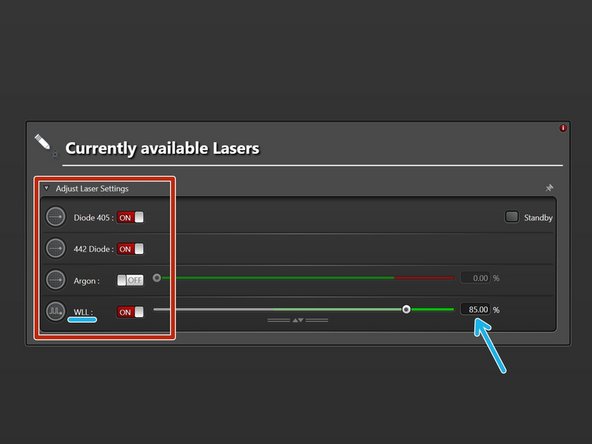
Switch "ON" the lasers you will need.
-
WLL (covers the excitation range from 470 nm to 670 nm). Power is set to 85% by default.
-
Argon laser. If needed, set power to 20%.
-
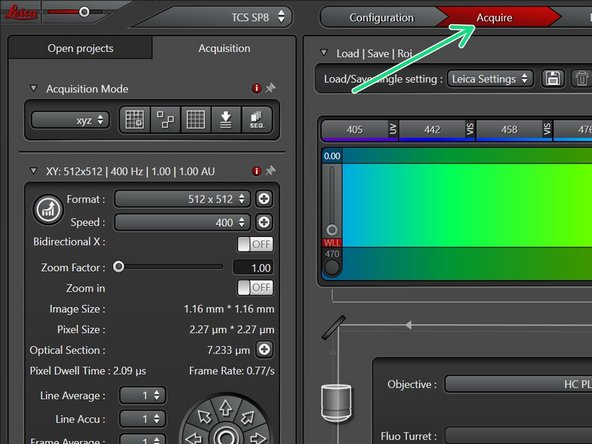
Go back to "Acquire".
-
-
-
Lower the objective turret by pressing the "Z" downwards button on the right side of the microscope.
-
This step avoids possible collision during placing of inserts and/or samples.
-
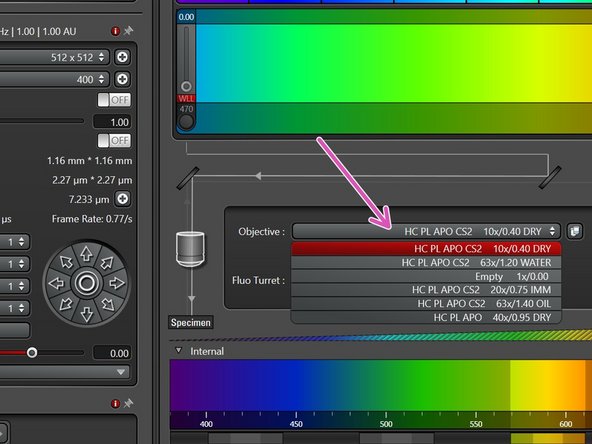
Select the 10x dry objective via the "LAS X" software.
-
In order to facilitate the focusing it is recommended to start with the 10x dry objective.
-
-
-
Push back the condenser arm.
-
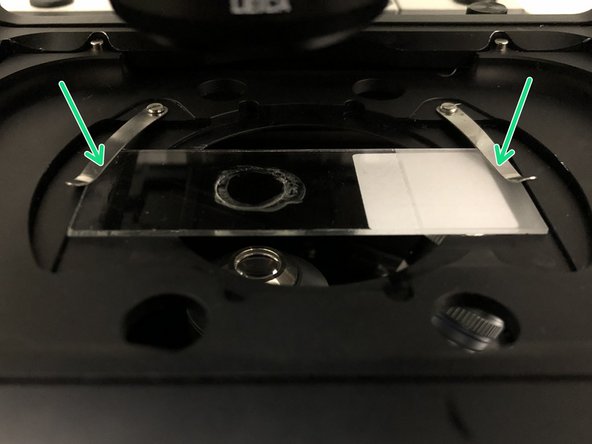
Insert your sample with the coverslip facing down and fix it with the two springs.
-
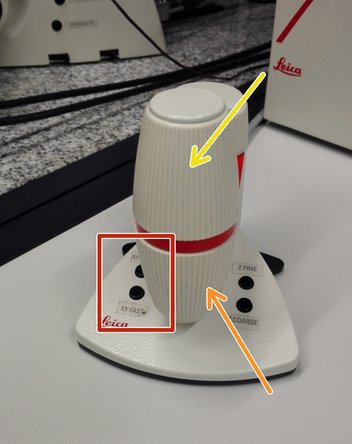
Move your sample above the objective with the help of the external controller "Smart Move".
-
Movement in y-direction.
-
Movement in x-direction.
-
Toggle between coarse movement "XY Fast" and slow movement "XY Precise".
-
Pull back the condenser arm to its straight position.
-
-
-
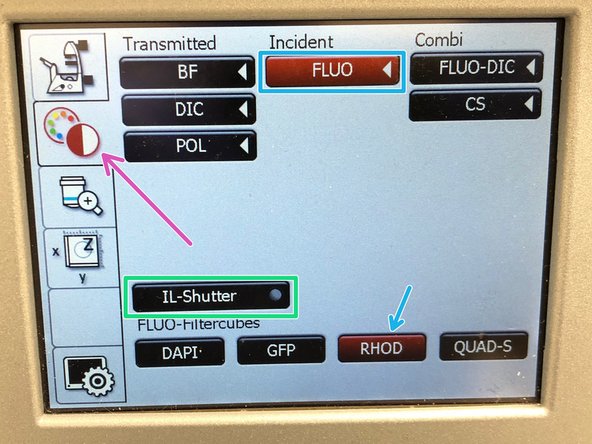
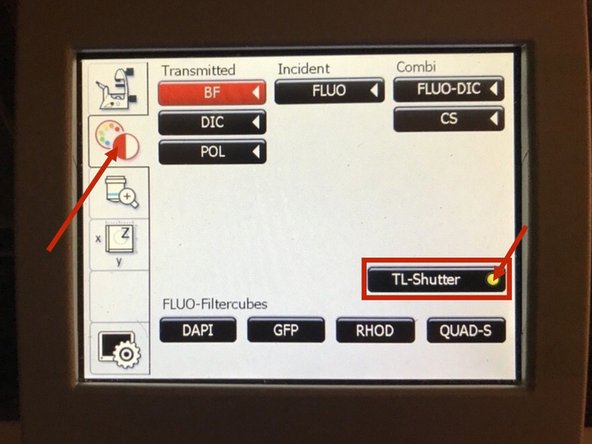
On the touch screen at the microscope stand choose the light path tab.
-
Click "FLUO" and choose an appropriate "FLUO-Filtercube" : e.g. "RHOD".
-
Open the "IL -Shutter" (if activated the dot is yellow).
-
Look through the oculars and focus your sample by using:
-
the focus wheel on the microscope stand,
-
or the z-wheel on the external controller ("Smart Move").
-
Turn z-wheels clockwise to move objectives upwards (closer to the sample). Turn z-wheels counter-clockwise to move objectives downwards (away from sample).
-
On the "Smart Move" toggle between "Z FINE" and "Z COARSE" to change the focusing speed.
-
-
-
In case you cannot find your sample with brightfield, please make sure:
-
1) The TL shutter is activated.
-
2) The "STOP" shutter is down. If it is in the upper position, it will block the beampath.
-
On a slightly different note, if the cable is going up, please push it down again (without touching the objectives!).
-
-
-
Storing the focus position is helpful in order to find the focus back if the sample or objective are changed.
-
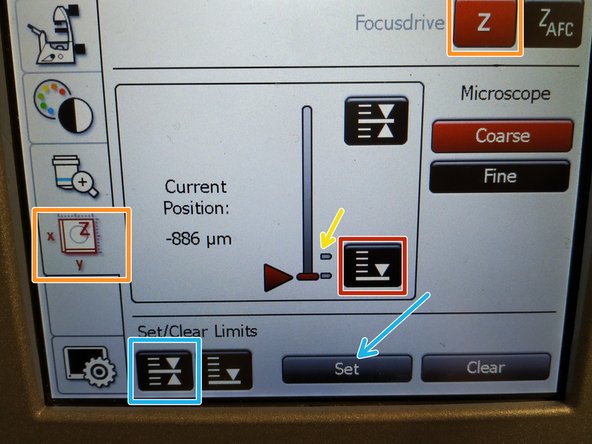
To save your current focus position select the "xyz tab" and the "Focusdrive Z" on the touchscreen of the microscope.
-
Click the "Upper Focus Limit" button.
-
Press "Set".
-
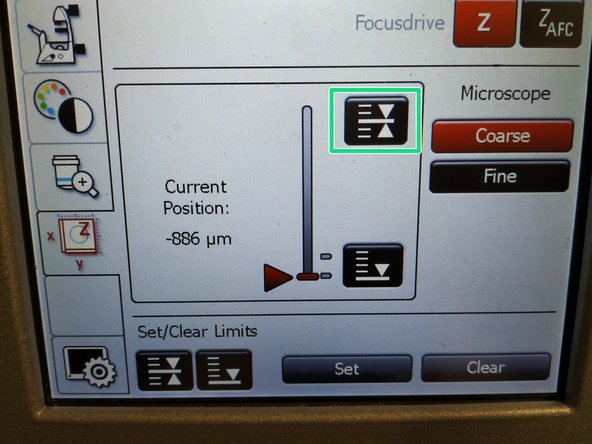
If done successfully you will see an upper marker line appearing.
-
Press the "Lower Limit" button in order to move down (for safe change of the objective or the sample).
-
-
-
Remove your sample and choose the objective of choice within the software.
-
Depending on the objective different immersion media have to be used. Apply directly on the sample.
-
Oil objectives: "Type-F" immersion liquid.
-
Glycerin objectives: either"Type-G" immersion liquid (for RT measurements) or "Glycerin" immersion liquid ''(for measurements at 37°C).
-
Water objectives : fresh ddH2O.
-
Please consider the additional information in the next step to guaranty proper image acquisition.
-
Mount your sample again and press the "Upper Focus Limit" button.
-
Focus your sample as described previously.
-
-
-
For optimal imaging performance on some objectives the correction collar has to be adjusted.
-
20x IMM (multi-immersion - Oil, Glycerin or Water) needs to be set to the corresponding immersion media ("OIL", "GLYC" or "0.17-W" (with cover glass) or "W-0" (without cover glass)).
-
40x dry you can correct for the cover glass thickness (0.11-0.23 mm). Standard is usually 0.17 mm.
-
63x Water is equipped with a motorized correction collar (motCORR) which can be adjusted via the "LAS X" software.
-
Make sure that the cap of the spring-loaded front lens is released (working position). Mandatory for all immersion objectives.
-
Please, DO NOT remove the objectives for adjustment. They can be directly accessed on the system.
-
Cancel: I did not complete this guide.
One other person completed this guide.